Coronavirus August 2020—Part 9 Masks, Vaccines, and Rapid Testing
Your COVID-19 Questions Answered—Rapid Tests, Masks, and Vaccines
In this post, we tackle the issue of masks, vaccines, and rapid testing for COVID-19. As always, our aim is to provide readers an evidence-based guide upon which to base their behavior and with which policymakers can determine policy. As usual, if you’re less interested in how we got to our conclusions than you are in the conclusions themselves, feel free to skip to the BOTTOM LINE in each section.
MASKS
Question: Will the wearing of masks in appropriate circumstances slow the pandemic?
Answer: Probably.
A review paper summarizing the scientific literature to-date on the use of masks to prevent the spread of viral infection found the following:
- Healthcare workers wearing respirator masks (N95 masks) are only protected from being infected by viruses when they wear respirator masks for an entire shift (compared to wearing them in a targeted fashion only during high-risk procedures such as bronchoscopy)
- Surgical masks do not reliably protect healthcare workers from acquiring viral infections from symptomatic patients even when worn for an entire shift
- A randomized, controlled trial of nine influenza patients showed that when infected subjects wore surgical masks and coughed into culture media, influenza failed to grow
- A large, randomized, controlled trial of patients with influenza-like illnesses wearing surgical masks showed no effect of masks when the data was analyzed in an intention-to-treat analysis (because many subjects didn’t wear their masks as instructed) but did show a reduced rate of infections in households where infected patients actually wore masks compared to households where infected patients didn’t wear masks (despite their mask assignment group).
A study also looked at which type of mask best prevent the spread of respiratory droplets (arbitrarily defined as particles > 5 micrometers). They used a laser, a cell phone camera, and a computer algorithm to measure the number of droplets spread when a speaker wearing different masks (and with different speakers wearing different masks) spoke the sentence, “Stay healthy, people.” Not surprisingly, the study found that filtered N95 masks (without an expiratory valve) did the best job, letting out almost no respiratory droplets at all. Surgical masks were a close second (superior, by the way, to vented N95 masks, which are designed to protect the wearer only). Most masks did a reasonably good job, with the exceptions of knitted masks, bandanas, and fleece. Note that there were no clinical endpoints in this study, meaning, no measure of how well each of these masks actually prevented infection (which is inferred to be in proportion to their ability to limit respiratory droplet spread, which, while reasonable to assume, may or may not be true). In the absence of clinical endpoints, it’s reasonable to conclude that most masks lower the risk of infection, with only fleece masks being equal to (or perhaps even worse) than wearing no mask at all. BOTTOM LINE: other than N95 masks, which aren’t readily available to the general public, surgical masks are best for non-medical personnel to use to prevent infection of others, but most other masks seem to be about as good.
Another study looked at viral RNA shed in respiratory droplets (again, defined as particles > 5 micrometers) and aerosols (defined as particles < 5 micrometers) in infected subjects wearing surgical masks vs. not wearing surgical masks. Three different viruses were studied: seasonal coronavirus (not SARS-CoV-2 but perhaps similar), influenza, and rhinovirus (one cause of the common cold). In the air around infected patients not wearing surgical masks, the investigators detected seasonal coronavirus RNA in respiratory droplets and aerosols in 30 percent and 40 percent of samples, respectively. In the air around infected patients wearing surgical masks, they detected no coronavirus RNA at all. The difference wasn’t quite statistically significant for respiratory droplets, but it was for aerosols. The participants were all coughing during the 30 minutes that the air around them was being sampled—except for four of the patients infected with seasonal coronavirus, who didn’t cough at all and around whom also no viral RNA was detected in either respiratory droplets or aerosols even in patients not wearing masks. Though this was a very small number, it suggests that patients infected with seasonal coronavirus aren’t likely to spread the virus into the air by merely breathing, nor are they likely to spread it into the air while coughing if wearing a mask. One important caveat: though seasonal coronavirus is similar in structure to SARS-CoV-2, we don’t know for certain that the two viruses spread into the air in the same way. Indeed, in this study, the results were different for all the viruses.
While all the studies mentioned above provide strong indirect evidence that if you’re symptomatic with COVID-19, wearing a mask is highly likely to prevent you from spreading the infection to others, none of them prove that mask-wearing by asymptomatic patients will slow the pandemic. Do we have any evidence that wearing masks in the right circumstances (indoors when people remain in close contact for extended periods or outdoors when you can’t achieve appropriate social distance) by asymptomatically infected people prevents enough super-spreader events to slow the pandemic?
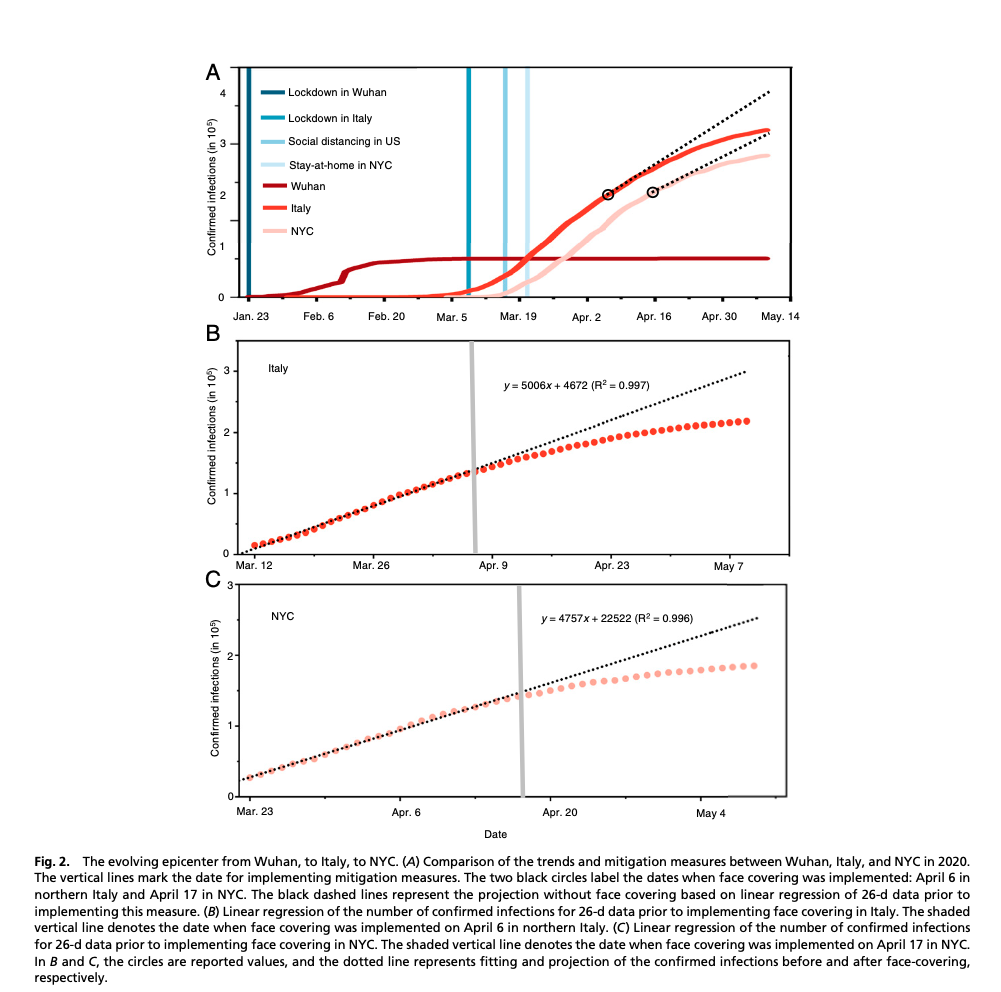
One study suggests that this is indeed the case. This study interpreted the differences in pandemic trends in relation to the mitigation strategies used in different parts of the world at different times. The study begins by noting that the flattening of the curve in China was potentially attributable to testing, quarantine, contact tracing, lockdowns, and mandated face mask-wearing in public. Unfortunately, all of these measures were instituted simultaneously, so there was no way to separate out the impact of each. In Italy and New York City, however, the mandate to wear masks came out after the mandates to quarantine, contact trace, and lock the economy down (in Italy, on April 6th and in NYC on April 17th). Prior to the mask mandate in Italy and NYC, the log of rates of infection and fatalities were linear. Only following the mask mandates in both Italy and NYC, compared to the rest of the U.S. and the rest of the world, did that linearity begin to drop off with a lag time of about 2 weeks (as we might have expected if mask-wearing was the cause), as can be seen in the figure below (the circles in graph A represent the point at which the mask mandates came out in Italy and NYC, nearly a month after a lockdown was instituted in each case) (data for the rest of the U.S.’s continuing linearity of infections and fatalities not shown):
Unfortunately, given its retrospective observational design, this study can’t prove that mask-wearing by asymptomatically infected people causes a reduction in the number of infections of COVID-19, but it identifies a strong correlation between the two.
Importantly, another study (study appendix here) that compared rates of infection in 15 states in the U.S. plus Washington, D.C., which all mandated mask-wearing between April 8th and May 15th, to rates of infection in states that didn’t have a mask mandate showed similar findings: a 2 percent decline in the increase of cases twenty-one days after the mandates were signed relative to states without mandates. Over time, this cumulative effect would be expected to become quite large. Though this was another retrospective observational study, they controlled for population density, the proportion of elderly, poverty rate, and a wide range of other variables. Importantly, the study wasn’t able to measure compliance with the mandate but assumed in states where mandates were in effect that mask-wearing was significantly more common.
BOTTOM LINE: The only way we’ll ever know for certain if mask-wearing by asymptomatic people, in the right circumstances, will reduce the spread of SARS-CoV-2 would be to prospectively assign a region (e.g., a city) to wear masks and compare its rates of infection over the same time to another region where people were assigned not to wear masks (and measure the compliance of each). The impossibility of conducting such a study at this point is obvious. Therefore, we’ll likely never be able to conclude with 100 percent certainty that mask-wearing in the right circumstances will slow the progression of the pandemic. But when we consider the sum of the evidence above, we conclude that mask-wearing by asymptomatic people, in the right circumstances, is likely to slow the progression of the pandemic.
Question: Who should wear masks, then?
Answer: Everybody.
The problem is that most of us who are asymptomatic don’t really believe we have the virus. And statistically speaking, we’re likely to be correct. The prevalence of asymptomatic infection is equal to the total number of people without infectious symptoms who nevertheless have COVID-19 divided by the total number of people without infectious symptoms. The total number of people without infectious symptoms who nevertheless have COVID-19—our numerator—we estimated in previous posts as being equal to the same number of people with infectious symptoms who have COVID-19, based on the data from the Diamond Princess study, which would currently be 5.2M (other studies have since suggested the number of asymptomatic infections is a lower percentage of symptomatic cases, but we want the worst-case scenario for purposes of estimating the risk of being asymptomatically infected). The total number of people without infectious symptoms—our denominator—would then be equal to the population of the U.S. (331M) minus the total number of people with symptomatic COVID-19 infections. As we wrote in an earlier post, that latter number is at best an estimate as we’ve been under testing the population. In a previous post, we assumed the number of symptomatic COVID-19 patients who haven’t been tested to be ten times the number of symptomatic COVID-19 patients who have been tested, which, as of this writing, would, therefore, be 52M people. The total number of symptomatic COVID-19 patients in the U.S. as of this writing then would be 52M + 5.2M (symptomatic, tested patients) = 57.9M symptomatic COVID-19 patients. The number of total asymptomatic patients in the U.S. not infected with SARS-CoV-2 would then be 331M – 57.9M = 273.1M. As of this writing, then, in the U.S., the prevalence of asymptomatic COVID-19 infections would be 57.9M divided by 273.1M x 100, which yields a prevalence of 21 percent. However, keep in mind that this number reflects the total number of cases we’ve had in the U.S. since the pandemic began, not the total number of cases we have right now. Thus, 21% is the likely upper limit of asymptomatic cases currently in the U.S., and likely by a wide margin.
This means if you’re asymptomatic, at the time of this writing, you have at least a 79 percent chance of not having COVID-19. Couple this fact with the data from contact tracing studies that suggest the risk of transmitting SARS-CoV-2 to close contacts while asymptomatic is approximately 0.33 percent (the study from which this number is taken doesn’t mention, unfortunately, if these asymptomatic people were wearing masks or not; we presume they weren’t, though, as they didn’t know until they entered the study that they’d been asymptomatically infected, but it’s not clear). Incidentally, from another contact tracing study, the risk of symptomatic transmission from casual contact outside of household and family member contact was even lower, 0.1 percent.
When you consider this data together, you have what seems on the surface to be a good argument for not wearing masks to reduce the spread of COVID-19 if you’re asymptomatic.
BOTTOM LINE: But it’s not. Here’s why: a 21 percent prevalence of asymptomatic SARS-CoV-2 infection represents 57.9M people infected. If each of those 57.9M infected people has a 0.33 percent chance of spreading the infection without wearing a mask and does so, it would amount to roughly 191,070 transmitted infections! (Even if our estimate of the number of asymptomatic infections is off by a factor of 10, this would still amount to 19,107 infections.) We don’t know to what degree wearing a mask will decrease the risk down from 0.33 percent, but even a small amount would translate into a large number of people. Thus, while the impact of one asymptomatically infected person not wearing a mask is small, the impact of all asymptomatically infected people not wearing masks may be large. The logic of collective action requires that individuals act as if their contribution is greater than it is because only that way do enough individuals act in such a way that yields the protection society needs. We all need to tolerate inconvenience to contribute to the greater good.
IMMUNIZATION
Question: Will we have a safe, effective vaccine for COVID-19, and if so, when?
Answer: Probably. But likely not until the first quarter of 2021 at the very earliest.
Immunization is one of the most effective—and safest—public health measures that exist. The prevalence of infections from diseases for which we now vaccinate children has declined by ninety percent (see chart below of effectiveness of routine childhood vaccinations). There are literally no other interventions in medicine that are as effective as vaccination.

* The time period for this graph precedes the measles outbreaks that occurred across the United States during and after 2008.
¶ Incidence per 100,000 children under five years of age.
_____
To understand why it will likely take us at least until the beginning of 2021 to have a safe, effective vaccine, you first have to understand a little about how vaccines are made, tested, and deployed.
In general, vaccines cause the body to generate an immune response to an infectious disease, which then protects people from developing it when subsequently exposed.
Scientists make vaccines by using several different strategies. First, they use weakened viruses that can’t easily reproduce inside the body. Examples of immunizations that use this strategy are measles, mumps, and rubella. These are called “live” or “attenuated” vaccines. One advantage is that they’re extremely effective: one or two doses provide immunity that’s usually lifelong. One disadvantage is that you can’t give them to people with weakened immune systems because it risks infecting them with the disease.
Scientists also make vaccines by using fully inactivated, or killed, virus. These immunizations cannot infect patients. Examples of immunizations that use this strategy are hepatitis A and the influenza shot. The advantage of this strategy is that these vaccines can be given even to people with weakened immune systems. The disadvantage is that they often require multiple doses to produce long-lasting immunity.
A third way scientists make vaccines is by using only part of a virus. This strategy can be used when an immune response to only one part of the virus is responsible for the generation of immunity. Examples of immunizations that use this strategy are hepatitis B and shingles vaccines. The advantage of immunizations that use this strategy is that they can be given to people with weakened immune systems and typically only require two doses to provide long-term immunity.
A fourth way scientists make vaccines—these against bacterial infections—is by inactivating the toxins such bacteria release, turning them into toxoids, which are then injected and induce immunity. Diphtheria and tetanus vaccines are made this way. Like inactivated viral vaccines, these bacterial vaccines can be given to people with weakened immune systems because they’re incapable of causing disease but often require multiple doses to induce long-term immunity.
There are other well-established strategies used to make vaccines as well and some experimental strategies, including using RNA vaccines where a piece of virus RNA is used to generate an immune response.
It usually takes ten years to move from the beginning of vaccine development to the widespread immunization of a population. Unfortunately, the failure rate of vaccines in development is higher than 90 percent. Once a vaccine has been developed, it’s tested in three phases.
Phase I trials represent the first time a vaccine is given to humans and is begun after animal trials have shown the vaccine creates an immune response in animals. In Phase I trials, the vaccine is given to a small number of people (a dozen or so) to measure its ability to produce an immune response and to look for harmful side effects.
In Phase II trials, the vaccine is given to more people (a few hundred to a thousand) to test for a consistency of an immune response and to monitor for adverse effects that weren’t seen in the Phase I trial. This phase is also used to work out the best dose and dosing schedule for the vaccine. These trials are typically randomized and placebo-controlled. They sometimes give some information on vaccine efficacy if human challenge trials are included. A human challenge trial (also called a Phase IIa trial) is one in which subjects are deliberately infected with the infection for which they’ve been immunized to see how often and to what degree they’re protected from becoming ill with it. Human challenge trials have only been done with diseases that aren’t fatal and when available treatments are known to cure it, such as malaria, typhoid, and cholera.
In Phase III trials, the vaccine is given to tens of thousands of people to observe for rarer adverse reactions and to test if the vaccine actually prevents immunized subjects from contracting the disease. This is done by vaccinating volunteers in hot spot areas of disease and then observing over time what percentage of vaccinated subjects contract the disease compared to the percentage of subjects given a placebo who contract the disease. Often, multiple trials that test different populations of subjects in different circumstances are needed (e.g., infants vs. older patients with co-morbid diseases). The development of a robust antibody response to a vaccine is a good thing but doesn’t guarantee immunity from the disease. This is why real-world Phase III trials are needed. Currently, one Phase III trial of an RNA vaccine is underway, with plans to vaccinate 30,000 people. It’s a placebo-controlled, double-blinded trial. It’s a two-shot series vaccination spread apart by 28 days.
Phase IV trials happen after the vaccine is given to the population at large and consists of monitoring for even rarer adverse reactions.
BOTTOM LINE: Currently, there are over 140 COVID-19 vaccines in development. Given the statistics we quoted above, that means we should end up with 14 viable vaccines. There’s one RNA vaccine being tested in a Phase III trial right now with 30,000 volunteers being given the vaccine. But we predict it will take us at least until early 2021 to determine if it’s a winner because it will take at least that long to make sure the vaccine is safe and effective. Remember, the risk of adverse reactions to vaccines needs to be substantially lower than the risk of adverse reactions to medications. This is because: 1) the number of people vaccinated will be much greater than the number of people given a medication (medications for diseases are given to at most millions of people; a vaccine for COVID-19 will be given to billions of people), so even small risks of harm can mean harm is done to millions of people, and 2) the vaccines are given to healthy people, not people already suffering from a disease. Thus, the risk of adverse events from the vaccine must be compared to the risk of not just contracting the disease but of experiencing a severe adverse outcome (i.e., severe, chronic morbidity or death). So, in the case of COVID-19, if we’re considering immunizing a 12-year-old child, for example, whose risk of dying from COVID-19 is literally only 0.022 percent, the risk of a severe adverse reaction to the vaccine needs to be far below that.
Unfortunately, the history of vaccine development is replete with stories of harm. One vaccine developed against respiratory syncytial virus (RSV) in the 1960s actually caused a form of immune enhancement where the disease was worse in vaccinated children, even killing two who’d been vaccinated. In 1955, Cutter Laboratories, a small pharmaceutical company that manufactured a polio vaccine, released vaccines contaminated with fully live virus due to manufacturing errors and poor government oversight, resulting in an estimated 40,000 children being infected with polio. Two hundred victims were permanently paralyzed, and ten of them died.
We mention these cautionary tales not to add fuel the anti-vaccine movement fire, but to highlight the importance of doing the science correctly, of not rushing inadequately tested vaccines to market. The reason vaccines are among the safest of medical interventions available is because they undergo such long and rigorous safety testing. As candidates come off the pipeline, we’ll review their efficacy and safety data and make recommendations about them.
RAPID VIRAL TESTS
Question: Are rapid tests worth doing, especially because the results are returned so much more rapidly?
Answer: Only if the rapid test comes back positive can you believe it.
The rapid viral detection tests so far have a very high false-negative rate as determined in one study—as high as 54.3 to 88.9 percent, depending on the site tested (sputum vs. nose) and collection method used. This means that if the test is negative, it tells you nothing, and you still have to get a PCR test. While the false-positive rates weren’t mentioned in this paper, in another paper, it was zero percent, meaning there were no false positives with the rapid test they used (which was different from the one used in the first paper cited above). In an asymptomatic patient, then, a positive rapid test would have a positive predictive value of 100 percent, meaning it could be believed. Unfortunately, other rapid viral detection tests may have a higher false-positive rate.
BOTTOM LINE: We don’t recommend people get a rapid test. Even though results take longer with the PCR test, a negative PCR test is more likely to be accurate. We know only one rapid test with a zero false-positive rate. Other rapid tests may not perform nearly as well.
For previous posts related to COVID-19, see:
- Coronavirus February 2020—Part 1 What We Know So Far
- Coronavirus March 2020—Part 2 Measures to Protect Yourself
- Supporting Employee Health During the Coronavirus Pandemic
- Coronavirus March 2020—Part 3 Symptoms and Risks
- Coronavirus March 2020—Part 4 The Truth about Hydroxychloroquine
- Coronavirus April 2020—Part 5 The Real Risk of Death
- Coronavirus April 2020—Part 6 Evaluating Diagnostic Tests
- Coronavirus April 2020—Part 7 The Accuracy of Our Antibody Test
- Coronavirus May 2020—Part 8 How to Reopen a Business Safely
[jetpack_subscription_form title=” subscribe_text=’Sign up to get notified when a new blog post has been published.’ subscribe_button=’Sign Me Up’ show_subscribers_total=’0′]
Epic. Thank you, sir!
Fabulous, comprehensive, informative, ultra-professional (as usual). Thank you for all this information.
The leading statistic (if that is the term) to understand and make projections of this pandemic is “deaths.” However, in some states (see below), about half the deaths are attributed to “underlying conditions” or “previous hospitalization.” The other layer of this is age—there is a clear distinction between, e.g., 30 years old and 70+ years old (also see below). There is no more detail I can find, e.g., which problems are in the “underlying conditions”?
I know that’s a lot of setup, so all I’ll ask is if there’s any insight to the “underlying conditions” category—can we infer anything? Does a chronic disease count, perhaps a cancer that’s in remission?
Thanks a lot for providing this forum!
Reference:
https://www.mass.gov/doc/covid-19-dashboard-august-1-2020/download
^^^there is an entry for yesterday (the 24th) but they – wouldn’t you know it – removed the age distribution I just found out today, not paying attention.
“Certain underlying conditions have been associated with a greater risk of death from COVID-19.”
Is that to say that other underlying conditions are not? Or that there is a fraction of deaths entirely due to COVID-19? Is there a condition with the highest risk factor?
“Cancer in remission isn’t one of them.”
That is interesting. Is there a list of the conditions with risk factors somewhere? I wonder if we can infer anything about the general health of a population from the data – for instance, obesity is high in the United States – is this evident in the affect COVID-19 is having on the population?
Obviously I have too many questions, but thanks for the help anyway!
Thanks, as always, for your sound insights and thorough analysis. What is your opinion regarding saliva based covid19 tests?
https://www.nytimes.com/2020/10/01/health/coronavirus-saliva-tests.amp.html